Marijuana Company of America MCOA Intro

Marijuana Company of America MCOA is looking very interesting, take a look!
Marijuana Company of America Company Summary
Company Name: Marijuana Company of America
Ticker: MCOA
Exchange: NASDAQ
Website: https://www.marijuanacompanyofamerica.com/
MCOA Company Summary
Marijuana Company of America (MCOA) invests in the cannabis sector directly. The company’s operations include C-Distro, one of the THC, Hemp & CBD cannabis industries fastest growing distribution companies, and hempsmart™, a Premium CBD company. The company’s core mission is to leverage its experience, and access to capital to identify and invest in acquisitions with unique growth potential.
Why did Marijuana Company of America go up?
Dec. 15, 2021
Announced that it has recently completed the acquisition of VBF Brands, Inc. (“VBF”) a marijuana cultivator and distributor based in Salinas, California. VBF was previously a wholly-owned subsidiary of Sunset Island Group, Inc. (OTC: SIGO).
MCOA 1 Day Chart
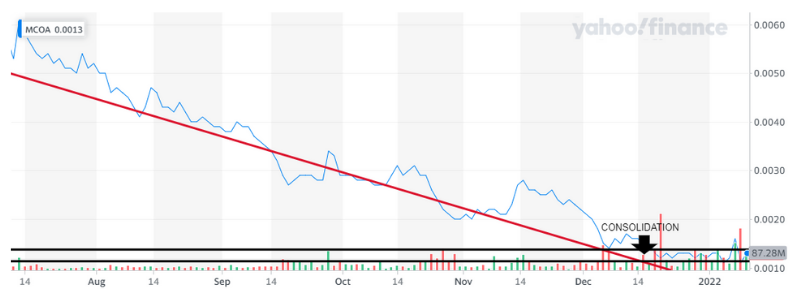
MCOA Technical Analysis
MCOA has been on a major slide for over a month. It appears that it is consolidating and it may be ready to move once we see a double confirmation. We just got one this week the next confirmation will be $.0017, keep a keen eye on that price target. It could take off once it crosses it.
GWPH Long Term Major Player in Medical Cannabis (NASDAQ:GWPH)

Intro & History:
GWPH, GW Pharmaceuticals (NASDAQ: GWPH) is a British biopharmaceutical company known for its multiple sclerosis treatment product Sativex, and Epidiolex (cannabidiol) which is their lead development drug nearing market introduction to treat a rare form of childhood-onset epilepsy which currently has few, if any, real treatment options. Sativex was the first natural cannabis plant derivative to gain market approval in any country. Founded in 1998 and based in Salisbury, UK GWPH is headed by Geoffrey W. Guy and Brian Whittle. GWPH calls themselves the “global leader in developing cannabinoid-based medicines.” We think that the epilepsy market is a sweet spot for cannabinoids because of relaxing medical marijuana regulations and the increasing prevalence of various types of epilepsy, all over the world.
Recent News & Developments:
On May 24th of this year, GWPH through its subsidiary Greenwich Biosciences published a “groundbreaking” study of Epidiolex in the prestigious New England Journal of Medicine. This corresponded with a slight surge in stock price, but came nowhere near historical highs. In any case, the current Phase III study has received ample positive attention not only in the NEJoM but from physicians specializing in conditions it is designed to treat at the American Academy of Neurology annual meeting on April 25 of this year.
This is starting to sound repetitive, but we have to say again that with relaxation of regulations at the state level, the federal government will eventually have to follow suit. So far that has remained elusive, especially with notorious drug warrior Jeff Sessions, the current U.S. Attorney General pressing Congress for the repeal of the Rohrabacher-Farr Amendment which prevents the federal government from prosecuting (or persecuting) marijuana based businesses that are in compliance with their state and local laws.
In some good news to counter the bad, Cory Booker, a liberal Democrat from New Jersey; Kirsten Gillibrand, a more moderate Democrat from New York; and Rand Paul, a libertarian leaning Republican from Kentucky introduced the CARERS Act which directly benefits firms like GW Pharmaceuticals by officially codifying Obama era policies whereby the feds largely ignored medical marijuana based businesses, and expanding opportunities for medical and scientific research into therapeutic uses for cannabinoids through a combination of banking, veterans access, and perhaps most importantly rescheduling marijuana away from the Schedule I designation which it shares with Heroin and LSD, making it very cumbersome to perform research. Finally, this act would completely remove cannabidiol (CBD), GWPH’s bread and butter so to speak, from federal drug schedules.
GWPH’s pipeline includes CBDV (GWP42006) for epilepsy and autism spectrum disorders and a host of other similarly cannabis-derived compounds for conditions including a form of neonatal encephalopathy, glioma, schizophrenia and complications stemming from multiple sclerosis. There are numerous clinical trial actions underway, viewable at the Clinicaltrials.gov website here.
Market Data & Performance:
Currently at $103.49 with a Market Cap of $2.61B. This is down from their all-time high of $132.73 in September of 2016 that coincided with the end of that fiscal quarter, the impending filing for FDA approval of Epidiolex (which still has yet to occur), and a resulting sharp spike of $50 in share price from August 1 to September 1, 2016. Since that time performance has been mostly negative, but could be looking up for Q3 2017.
Various positive indicators came out of the Q2 2017 call in early May. These included improving assets to liabilities and assets to inventory ratios. They’ve budgeted a significant $130M-$150M to spend for the next 12 month period, largely on operations, research and market penetration. They also discussed high profile Phase III research and development news regarding Epidiolex – which may finally be nearing FDA approval, several management changes including the hire of Scott Giacobello as their new CFO and plans to become a U.S. domestic NASDAQ registrant reporting under U.S. GAAP (and in U.S. dollars) in the near future, among other items.

Conclusion & Looking Ahead:
Based on current and historical performance, as well as positioning of current and future drug releases, GWPH is probably a bit undervalued. Sales of legal cannabis and marijuana in both the recreational and medical sectors are expected to rise by ~30% in 2017, ~45% in 2018, and reach $17 billion (or more) in 2021, per the “Marijuana Business Factbook 2017”. Numerous cannabis related stocks have already surpassed 2X or 3X on their value at introduction/IPO and GW is sitting at a pretty low point right now.
We think you are capable of doing the math here. While I cannot make any specific recommendations on this stock, you will be well served to short list a group of marijuana based stocks and follow them closely in the coming months and years. This should be one of them – keep an eye on upcoming clinical trials because the FDA and EMA processes to bring new drugs to market is long and fraught with peril and the floor can drop out at any time as a result. You should also be wary of a “green rush” bubble propping up marijuana based stocks but also understand that the inherent safety of cannabinoid products and derivatives, along with the aforementioned relaxed federal medical cannabis regulatory environment, do provide some insulation from sudden stock crashes resulting from suits or government “imminent public safety” action.
Finally, the status quo in Washington D.C. could be good for firms with existing research capabilities and market penetration like GW. Namely, recreational use is facing a steep climb toward federal legality, but numerous pieces of legislation currently making their way through Congress with the aim of easing restrictions on all aspects of the medical marijuana business.
ABBV AbbVie Inc Continues To Impress (NYSE:ABBV)

Intro & History on ABBV (NYSE:ABBV) :
In the biotech and medical marijuana arena, we think that ABBV AbbVie Inc. (NYSE ABBV) is an interesting and well known stock to keep an eye on. ABBV is a pharmaceutical development, discovery, manufacturing and sales firm based in Illinois near Chicago, and traded on NYSE and they are, according to Wikipedia, the world’s eighth-largest independent biotech company by market cap. AbbVie falls squarely into the “Big Pharma” category. From their own company literature they are:
“…a global, research-driven biopharmaceutical company committed to developing innovative advanced therapies for some of the world’s most complex and critical conditions. The company’s mission is to use its expertise, dedicated people and unique approach to innovation to markedly improve treatments across four primary therapeutic areas: immunology, oncology, virology and neuroscience. In more than 75 countries, AbbVie employees are working every day to advance health solutions for people around the world.”

AbbVie originated in 2013 when Abbott Laboratories decided to separate into two separately traded companies. Abbott is now focused only on medical devices, equipment and nutrition products whereas AbbVie is strictly in the pharmaceutical research and manufacturing business.
They manufacture several products, which in this day and age of ubiquitous pharma adverts, can arguably be called household names. The two most prominent of which – for our purposes anyway – are Humira and Marinol. Humira – This immunosuppressive drug accounts for over 60% of AbbVie’s revenue and is used to treat Crohn’s disease and happens to be the highest grossing drug *in the world*, with over 15% growth in the past year alone. There are so-called “patent cliff” concerns looming, but I’ll address those momentarily. Marinol – This marijuana derivative, also known as Dronabinol, is marketed to chemotherapy, AIDS and other patients dealing with nausea, vomiting and suppressed appetite resulting from a pathology or treatment regimen. It is currently regulated as a Schedule III substance under the Controlled Substance Act, differing markedly from marijuana which sits at Schedule I and carries unreasonably harsh criminal penalties in many places in the United States and abroad. Medicinal cannabis/marijuana is currently approved in over half of the United States.
Recent News & Developments:
ABBV currently has an alphabet soup of promising experimental drugs in the hopper. The one drawing the most publicity is Upadictinib (formerly research chemical ABT-494) which recently significantly outperformed a placebo in clinical trials for rheumatoid arthritis. The FDA recently rejected Baricitinib (developed and submitted by Eli Lilly and Incyte) thereby seriously limiting competition for Upadictinib. Now all AbbVie has got to do is find a catchier name for Ubpadictnib it and send out the sales force. I kid, of course; if history is any indication, it could be named “Thistuffakillyatinib” and it wouldn’t make a bit of difference – so long as it continues to work without any previously unforeseen large scale side effects and the accompanying class actions cropping up.
The one big question mark at this point; and one which doesn’t have too many informed investors and biotech experts worried given the timeframe, is the expiration of Humira’s patent in 2022 and the introduction of competing drugs. Since ABBV derives so much of their current income from this product, it is of course incumbent on them to develop replacement revenue drivers between now and then. Again, most analysts with whom I am familiar are bullish on the likelihood that this will indeed happen.
Market Data & Performance:
During the last one-year period this Large Cap stock has fluctuated between $55.06 (low) and $73.67 (high) with an average volume of 5,560,234, a current volume of over 9,000,000 and higher than average yield. The characteristic zigzag graph indicates rallies and reactions over the past five years, but the stock has trended generally upward in every year for which data is available since 2012. To further belabor the point, almost every new peak or trough has been higher than the preceding peak or trough through time, and the line-of-best-fit has a definitively positive slope. ABBV has used about 58% of free cash flow to cover their past four quarterly payments which isn’t ideal, but far from abnormal. Price closed at $73.18 on June 22, 2017 and is currently trending down, but only by about 1% on June 23. Other mostly current stats are as follows:
- Market Cap: 469B
- PE Ratio: 18.97
- EPS: 3.86
- 6% yield
The company released its first quarter 2017 financial results and outlook at the end of April and it contained the following statement:
AbbVie is confirming its GAAP diluted EPS guidance for the full-year 2017 of $4.55 to $4.65. AbbVie expects to deliver adjusted diluted EPS for the full-year 2017 of $5.44 to $5.54, representing growth of 13.9 percent at the mid-point. The company’s 2017 adjusted diluted EPS guidance excludes $0.89 per share of intangible asset amortization expense and other specified items.
Paying down of the relatively high debt load has not, and looks to continue to not be a priority, which could mean that the C-suite is confident in the likelihood of continued future growth.
Looking Ahead:
To those of you with a particular interest in the cannabis market sector, AbbVie has had Marinol since 1985 and has over 30 years of research into cannabinoid compounds. Several experts have speculated that one path away from ABBV disproportionately heavy reliance on Humira and the dangers associated with the impending collapse of its veritable monopoly, would be in medical marijuana derivatives – the market for which is in the process of moving from $5 Billion in 2015, to over $20 Billion by 2020.
Conclusion:
Humira is facing existential competition, but not anytime soon. Toward weaning themselves from Humira, ABBV acquired rights to ½ of sales of Imbruvica, a cancer drug, in 2015 and it is joined in their oncology drug portfolio by Venclexta another novel cancer treatment drug. There are, again, many more products in the research phase.
Even if there is an overall market correction in biotech, it would affect all such stocks equally and probably hit the smaller companies harder – and given the increasingly positive reception to relaxed medical (and recreational) marijuana laws and the tax revenues that decriminalization and legalization would bestow at the state and national levels, it would behoove AbbVie to seriously consider this market vertical for continued research, development, and acquisitions. They have the benefit of their extensive previous research and the notion that the DEA simply has to be trending toward totally re-scheduling marijuana (and its derivatives); and we feel like they can afford to be; actually – they NEED to be very aggressive in pursuing innovations in this market.
Further, given Humira’s continuing status as the #1 revenue grossing drug on the planet, the next 3 or so years look promising as a mid-term investment – depending on how proactive AbbVie can be in staking out aforementioned (or currently unannounced) new market footprint(s). Overall, in our opinion, the lower AbbVie trends toward the $60-65 threshold, the better the mid-to-long term bargain for investors and the more closely it should be followed.
Cara Therapeutics Continues to Climb (NASDAQ:CARA)


We think Cara Therapeutics (NASDAQ: CARA), a boutique “clinical stage” biotech/biopharma firm which develops novel compounds (including cannabis derived chemicals) for neuropathic pain is worthy of a close look. Cannabis regulation has been relaxing both globally and in the United States and this influences our stance on CARA stock. But there has also been positive recent product news. Furthermore, current trend analysis indicates that CARA could be on the cusp of becoming a real steady performer on the market.
Good news: The U.S. Food and Drug Administration (FDA) just granted “breakthrough therapy status” to Cara’s CR845, a unique therapeutic compound which has provided significant clinical benefits to some patients with chronic kidney disease and osteoarthritis (more on that in a bit) and trading has picked up significantly in recent days likely as a result.
Cara is based in Stamford, CT and was founded by Derek T. Chalmers, Michael E. Lewis, and Frédérique Menzaghi in 2004 (initial IPO on January 30 of that year) with the following mission:
… to fundamentally change the way acute pain, chronic pain and pruritus are managed. We aim to achieve this by developing new products that selectively target the body’s peripheral kappa opioid receptors. Cara is developing a novel and proprietary class of product candidates that target the body’s peripheral nervous system and have demonstrated initial efficacy in patients with moderate-to-severe pain and pruritus (itch) without inducing many of the undesirable side effects typically associated with currently available pain and itch therapeutics.
Recent News & Developments:
The FDA granted breakthrough therapy status for CR845, a drug for treating itching (uremic pruritis), which is an intractable and common (affecting up to 50% of patients) presenting symptom with chronic kidney disease patients undergoing hemodialysis. Currently CR845 is an intravenous drug, but an oral version is in late stage development. This is significant because of the lack of available effective systemic treatments that is compounded by a dearth of new product development by other pharmaceutical companies. Available treatment regimens span the gamut from oral antihistamines to topical creams, standard analgesics (NSAIDs) to risky ultraviolet light treatments which can cause skin cancers in individuals with fair complexions. CR845 showed a 68% reduction in worse itching scores and a 100% improvement to quality of life.
A quick note on the meaning of breakthrough therapy status: According to Wikipedia, breakthrough therapy is a United States Food and Drug Administration designation that expedites drug development that was created by Congress under Section 902 of the July 9, 2012 Food and Drug Administration Safety and Innovation Act. It allows the FDA to grant priority review to drug candidates if preliminary clinical trials indicate that the therapy may offer substantial treatment advantages over existing options for patients with serious or life-threatening diseases. The FDA will work with the sponsor of the drug application to expedite the approval process. This can include rolling reviews, smaller clinical trials, and alternative trial designs. In short, it simply means that 3-9 months are potentially shaved off of the standard review and approval cycle time.
Cara’s pipeline consists of CR845, variants upon CR845 and CR701, a chronic pain treatment which operates on peripheral cannabinoid receptors, particularly in immune cells such as leukocytes and mast cells, which have been shown to be involved in pain and inflammatory responses but do not affect cannabinoid receptors of the Central Nervous System (CNS). CR701 is an advanced compound presently in preclinical development for hyperalgesia (extreme sensitivity to pain) and allodynia (perception of harmless stimuli as pain) where it has been proven significantly effective in laboratory trials involving rodents.
Looking to extend their coverage abroad,Cara has in place licensing agreements with Chong Kun Dang Pharmaceutical Corporation to develop, manufacture, and commercialize products containing CR845 in South Korea; and Maruishi Pharmaceutical Co., Ltd to develop, manufacture, and commercialize drug products containing CR845 in Japan.
Market Data & Performance:
In 2017, Cara shares have risen a phenomenal 134+%, with stocks going for about $4.50 in July of 2016 trending all the way up to where they sat at $22.87 on Friday June 23, 2017. There was a slight dip in afterhours trading ending at $22.59 – but overall performance for CARA definitely defied the general biotech sector’s slide for the past month or so. Market Cap is $736.28M. Financials appear to be up-to-date and are listed at www.sec.gov or at their investor relations webpage. An EPS of $-2.52 might usually raise red flags, but given that we’re talking about a biotech – a sector in which firms lose money for years during the development of patient-ready products – CARA’s negative earnings per share is probably not a big deal.
Conclusion & Looking Ahead:
With the recent good news from the FDA and resulting trading performance, you can probably assume that EPS and share prices will improve in the near- to mid-term, but it will be important to keep an eye on how CARA manages its revenues and expenses moving forward.
Regarding the negative EPS, in Q1 2017 CARA managed to expend $22M, but the prospect of having its first product reach the market many months ahead of schedule means they have a good shot of reversing cashflow into the black sooner rather than later – and making a serious run at $30/share in the next six months. Of course there are well documented risks for retail investors in small cap biotech so it’s not wise to make such a stock the centerpiece of your portfolio or go in too heavy before the medical sector starts buying CARA’s products in quantity. We think this is a cannabis related stock to watch closely through the end of Q2 and into Q3, 2017.
Aurora Cannabis Inc. (OTCMKTS:ACBFF) ACBFF leading the way for Cannabis companies?

Today (OTCMKTS:ACBFF) Aurora Cannabis had gains of more than 7% on news that they will be making investments in Hempco HEMP.V
Both of these companies are leading the way in the cannabis industry. With growing interest from consumers and manufactures alike, hemp products are trending and investors are begging to be part of this movement.
VANCOUVER and BURNABY, BC , June 8, 2017 /CNW/ – Aurora Cannabis Inc. (the “Company” or “Aurora”) (ACB.V) (ACBFF) ( Frankfurt : 21P; WKN: A1C4WM) and Hempco Food and Fiber Inc. (“Hempco”) (HEMP.V) are pleased to announce that Aurora will be making a strategic investment in Hempco for an ownership stake of up to 19.9% on a fully diluted basis, subject to Regulatory and Board approvals, as well as satisfactory completion of due diligence. Additionally, subject to customary conditions, Aurora will obtain an option to acquire shares from the majority owners of Hempco that, upon exercise of the option, will bring Aurora’s total ownership interest in Hempco to 50.1% on a fully diluted basis.
This news brought hungry investors into both ACBFF and HEMP.V with gains in ACBFF of more than 7% and HEMP.V showing double digit gains around 14%.
Hempco is one of the world’s largest industrial producers of hemp and hemp products, and currently offers three primary product lines: (1) bulk and packaged food products (e.g. hemp protein powder, hemp seeds or hearts, hemp oil etc.); (2) hemp fibre; and (3) nutraceuticals. Hempco’s line of packaged foods are sold under the brand “Planet Hemp” and are distributed globally in seven countries.
The target market for these products includes, but is not limited to, health conscious consumers, including vegetarians seeking to supplement protein and reduce or eliminate animal product intake, as well as an increasing number of consumers focused on managing and preventing a variety of health issues through a healthy diet, known as “LOHAS”, Lifestyles of Health and Sustainability, and “millenials” looking for clean and “green” products.
In August of 2016 ACBFF was sitting around $0.35 a share but just a few months later it hit a high around $2.70 in the middle of November. Over the next 6 months ACBFF bounced between $1.70 and $2.65 with average volume over the last 30 days around 490,000 shares. Just in the last week some heavy than normal selling brought the PPC to a low of $1.50
We will continue to watch ACBFF over the short term and keep you updated on any changes that deserve your attention.
March Review Released for INVICTUS MD Strategies Corp (OTCMKTS:IVITF)

INVICTUS MD Strategies Corp (OTCMKTS:IVITF) shares ticked 9.75% higher to $1.27 on Wednesday and were flat in after-hours trading. Share prices have been trading in a 52-week range of $0.18 to $1.61. The company has a market cap of $43.50 million at 37.50 million shares outstanding.
INVICTUS MD Strategies Corp is a company that is focused on three main verticals within the burgeoning Canadian cannabis sector: Licensed Producers under the ACMPR including an investment in a fully licensed facility, AB Laboratories Inc. as well as the option to now acquire 100% of Acreage Pharms Ltd.; Fertilizer and Nutrients through Future Harvest Development Ltd.; and Cannabis Data and Delivery, with its wholly owned subsidiary Poda Technologies Ltd.
In a press release, INVICTUS MD Strategies Corp Chairman Dan Kriznic provided a month in review, highlighting the transformational time for the company as it worked to expand and solidify its cultivation portfolio with expansion plan forecasts of 50,000 kilograms of high quality cannabis production by 2020.
He started off by sharing that CBC News learned that marijuana could be legal in Canada by July 1, 2018 and that the legislation will be announced during the week of April 10. Analysts estimate that this industry could reach $22.6 billion over the coming years, according to Deloitte, with a retail market worth up to $8.7 billion. So far, there are only 41 producers licensed by Health Canada so there’s still significant potential for INVICTUS MD Strategies Corp to capitalize on this growth over time.
At the beginning of March, INVICTUS MD Strategies Corp announced that it had closed its previously announced bought deal private placement with Canaccord Genuity Corp. and Eventus Capital Corp., including a portion of the over-allotment option, for aggregate gross proceeds of $16,218,065. Soon after, the company completed its final commitment with a cash transaction of CAD$2,000,000 to acquire 33.33% of AB Laboratories Inc. This is a Licensed Producer under the Access to Cannabis for Medical Purposes Regulations in Ontario.
Towards the end of the month, the company closed the definitive option agreement to acquire 100% interest in OptionCo which had received its license to cultivate under the Access to Cannabis for Medical Purposes Regulations as Acreage Pharms Ltd. It has constructed a 6,800 square foot production facility with an expansion plan floor plate of 30,000 square feet, and the option to add a 20,000 square foot mezzanine on 150 acres of land in Alberta.
As one of the few cannabis producing companies in North America listed on a major exchange such as the TSXV, that move will enable us to engage a wider investor audience,” said Dan Kriznic, Chairman & CEO, Invictus MD. “We are strongly focused on building our shareholder value. With 250 acres of cultivation space that stretches from Alberta to Ontario, allowing for purpose built production facilities rather than retrofitting existing buildings, we now have the largest land package in Canada for building cultivation facilities as demand increases and we will continue the disciplined but agile execution of our business strategy,” added Kriznic.
DISCLAIMER: There is a substantial risk of loss with any speculative asset, especially small cap stocks. The opinions expressed are those of the author, and do not constitute recommendations to buy or sell a stock. Do your own research before committing capital.
22nd Century Group Inc (NYSEMKT:XXII) Shares Up 12.5%

22nd Century Group Inc (NYSEMKT:XXII) shares rose 12.5% on Thursday to $1.20 and were unchanged in after-hours trading. Share prices have been trading in a 52-week trading range of $0.73 to $1.71. The company has a market cap of $107.83 million at 90.70 million shares outstanding.
22nd Century Group Inc is a plant biotechnology company that is focused on technology that allows increasing or decreasing the level of nicotine and other nicotinic alkaloids in tobacco plants, and levels of cannabinoids in cannabis plants through genetic engineering and plant breeding.
It is engaged in various activities, including research and development of less harmful or modified risk tobacco products and tobacco plant varieties; development of X-22, a smoking cessation aid consisting of very low nicotine (VLN) cigarettes; manufacture, marketing and distribution of its RED SUN and MAGIC cigarettes; production of SPECTRUM research cigarettes for the National Institute on Drug Abuse (NIDA); contract manufacturing of third-party branded tobacco products, and research and development of plant varieties of hemp/cannabis, such as plants with low to no amounts of delta-9-tetrahydrocannabinol (THC), plants with high levels of cannabidiol (CBD), and other non-THC cannabinoids.
In a press release, 22nd Century Group Inc announced that representatives of the company’s wholly-owned subsidiary, Botanical Genetics, have been invited to serve as featured speakers and presenters at the annual Colorado Hemp Expo. This will be held at the Ranch Events Complex, 5260 Arena Circle, Loveland, Colorado on Friday, March 31, 2017, and Saturday, April 1, 2017.
We are developing several exciting new strains of medical marijuana as well as industrial hemp varieties with substantially altered levels of cannabinoids – all of which will be below the legal limit of 0.3% THC,” explained Dr. Paul Rushton, Vice President of Plant Biotechnology. “Following our recently announced success with Zero-THC hemp plants and our major collaboration with the University of Virginia, Botanical Genetics is now aggressively expanding our activities in an effort to make our Company a major source of proprietary plants for both the legal medical marijuana and industrial hemp markets. We look forward to exploring exciting business opportunities with like-minded companies in Colorado.”
Along with Thomas James Esq., Vice President & General Counsel, they will provide conference attendees with business and scientific updates and highlights of 22nd Century Group Inc advances in the rapidly evolving legal medical marijuana and industrial hemp markets. This includes Dr. Rushton’s featured speech on Friday evening regarding the 22nd Century Group Inc proprietary cannabis initiatives like their recently announced development of THC-free industrial hemp plants and the propagation of medical marijuana strains that are selected for increased levels of medically-important cannabinoids.
Dr. Rushton will also speak in a panel discussion on Seeds, Clones, Pesticides and Pollen while James will speak on three panel discussions: Investments in Hemp, Global Hemp Activities, and The Future of Hemp. They will also be available for one-on-one discussions.
DISCLAIMER: There is a substantial risk of loss with any speculative asset, especially small cap stocks. The opinions expressed are those of the author, and do not constitute recommendations to buy or sell a stock. Do your own research before committing capital.
ProText Mobility Inc (OTCMKTS:TXTM) Shares Drop on Latest Acquisition

ProText Mobility Inc (OTCMKTS:TXTM) shares fell 20.83% on Monday to $0.00190 and were unchanged in after-hours trading. Share prices have been trading in a 52-week range of $0.00 to $0.01. The company has a market cap of $5.92 million at 1.95 billion shares outstanding.
ProText Mobility Inc is a company that develops, markets and sells software solutions for the mobile communications market primarily for protecting children from dangers derived from mobile communications and mobile device use. Its offerings include solutions with downloadable applications for mobile communications devices, such as SafeText, DriveAlert and Compliant Wireless.
Its SafeText is a service for mobile devices that provides parents a tool to help manage their children’s mobile communication activities. Its DriveAlert is a virtual lock-box, designed to curb mobile device use while driving and to help mitigate the risks of driving while distracted. Its Compliant Wireless is a mobile platform designed for small to large companies. The mobile solutions for the enterprise/corporate compliance are marketed under Compliant Wireless and those with consumer solutions are marketed under FamilyMobileSafety.
In a press release, ProText Mobility Inc shared that it acquired Cannabis Biosciences Inc. from Plandaí Biotechnology Inc. In this transaction, ProText Mobility Inc acquired 100% of the capital stock of Cannabis Biosciences in exchange for 50 million shares of its common stock. According to Plandai, the shares will be distributed to Plandaí shareholders as a stock dividend and operate its two wholly owned subsidiaries as independent operating businesses.
“Where most pharma companies have chosen to focus on the non-psychoactive CBD chemical, we believe that this strategy limits the potential medical benefits. Recent third party studies have demonstrated the synergistic value of retaining the full chemical profile of cannabis. Our objective is to validate Cannabis Biosciences cannabis extracts to be not only full profile but also non-psychoactive, which will give researchers all of the benefits of cannabis without the unwanted side effects. The acquisition of Cannabis Biosciences by Protext properly aligns our pharmaceutical research under one roof, allowing us to further our cannabis studies as we strive to create a cannabis extract that can increase the well-being and potentially improve the lives of so many people,” said Roger Baylis-Duffield, CEO of ProText Mobility Inc.
Cannabis Biosciences was formed and incorporated by Plandaí Biotechnology in 2013 to legally develop non-psychoactive medicines from live cannabis plant using Plandaí’s proprietary processing and extraction technology. It intends to commence investigations in conjunction with independent researchers to develop and validate a full-profile cannabis extract, one that contains both CBD and the precursor acid form of THC found in the live cannabis plant.
In particular, the company’s investigations will be designed to show that the Cannabis Biosciences extraction process, which will use live leaf and low temperatures to extract the phyto-chemicals, should leave the acid forms of THC intact, resulting in a non-psychoactive extract with full medicinal potential.
DISCLAIMER: There is a substantial risk of loss with any speculative asset, especially small cap stocks. The opinions expressed are those of the author, and do not constitute recommendations to buy or sell a stock. Do your own research before committing capital.
